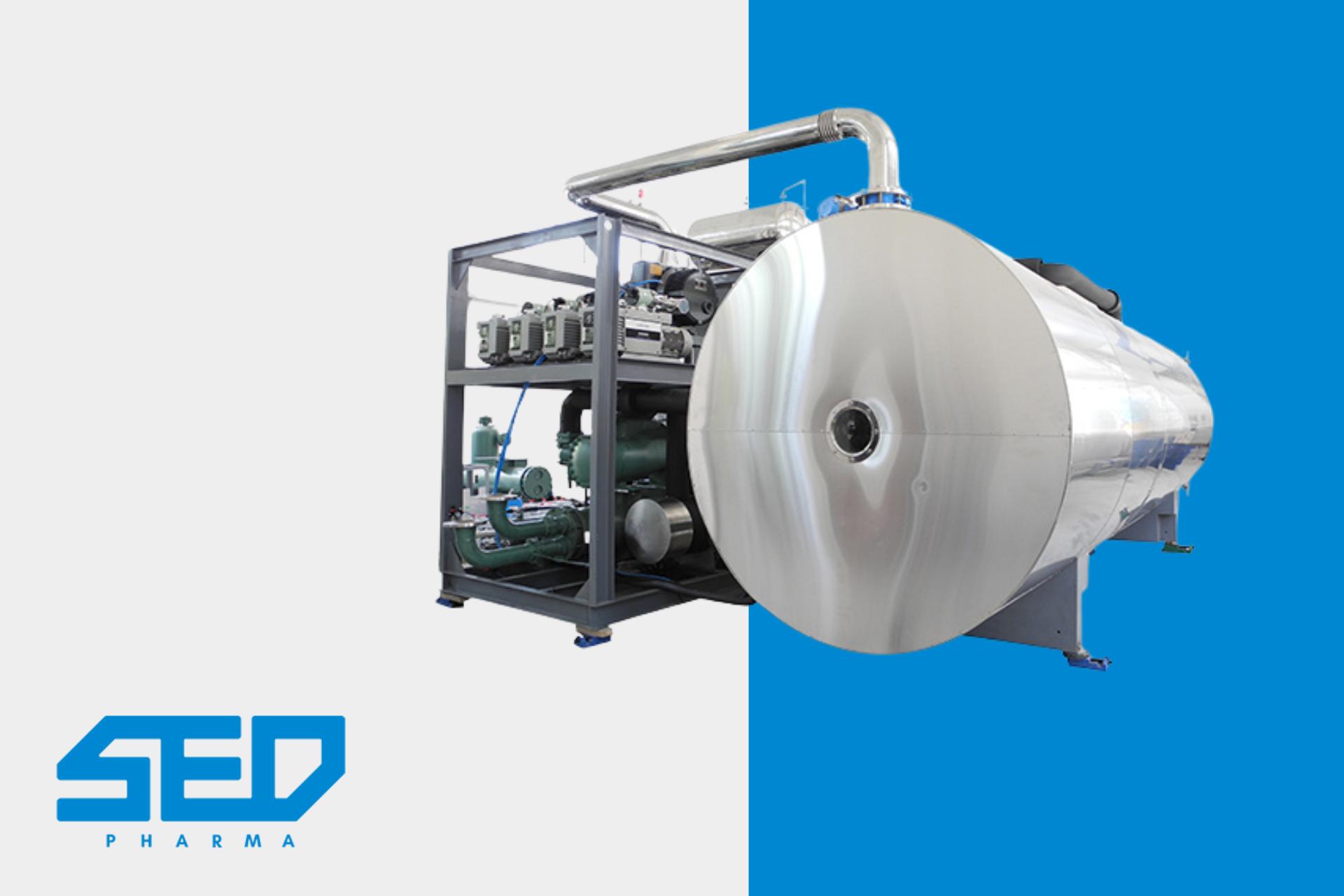
Freeze dryers play a pivotal role in pharmaceutical manufacturing, ensuring the stability and longevity of critical medical products. SED Pharma, a leading pharmaceutical equipment supplier, offers a range of advanced freeze-drying solutions.
Freeze dryer machines meticulously remove moisture from pharmaceuticals, preserving their biological structure and potency. The process, known as lyophilization, is essential for sensitive medications, vaccines, and biologics. It not only enhances shelf life but also ensures the drugs remain effective until they reach patients. SED Pharma’s expertise in designing and manufacturing these vital machines demonstrates our commitment to advancing pharmaceutical care and safety.
Some Background on Freeze Drying
Freeze drying, or lyophilization, is a sophisticated process integral to pharmaceutical manufacturing, known for its ability to preserve the integrity and efficacy of sensitive drugs. Freeze drying in pharmaceuticals involves three key stages:
- Freezing: The product is cooled below its triple point to ensure solidification. This step is crucial to maintain the product’s structure during drying.
- Primary Drying (Sublimation): Under a vacuum, ice transitions directly from solid to vapor, removing most of the water content without compromising the product’s structure or chemistry.
- Secondary Drying (Desorption): Residual moisture is gently removed by slightly raising the temperature, ensuring the stability and longevity of the product.
Each stage is precisely controlled to maintain the product’s integrity and effectiveness. Unlike conventional drying methods that involve heat, freeze drying preserves the biological and chemical profiles of drugs, making it ideal for temperature-sensitive pharmaceuticals.
This method is particularly beneficial for preserving biologics, vaccines, and other complex formulations, ensuring they remain effective and safe for use. By removing water without altering the fundamental biochemistry of the product, freeze drying extends the shelf life and simplifies storage and transportation. The precision and control offered by freeze drying technology are unmatched by other drying techniques, affirming its crucial role in modern pharmaceutical production.
SED Pharma are Leaders in Pharmaceutical Freeze Dryer Machines
The SED Pharma features a variety of freeze dryer machines in various models. These models are designed for a variety of applications, ranging from small-scale laboratory research to large-scale industrial production. Each model features unique specifications and capabilities, making them suitable for different needs in the pharmaceutical industry. For detailed descriptions of each model, please visit SED Pharma Freeze Dryers.
SED Pharma Announces a New California Showroom
SED Pharma has announced the opening of a new branch office in San Bernardino, California. This expansion will allow us to better serve our clients across North America. The new office will feature a showroom displaying their main products, including capsule counting lines and freeze dryers. Additionally, SED Pharma plans to offer services like machinery financing, training, installation, maintenance, and spare parts sales at this location. For more details, you can visit our announcement page here.
Shop At SED Pharma USA, Your Best Choice for Pharmaceutical Equipment
SED Pharma, customers can explore a diverse range of freeze dryers, each tailored to meet specific pharmaceutical manufacturing needs. Whether for small-scale laboratory research or large-scale industrial production, SED Pharma’s freeze dryers are designed for precision, efficiency, and reliability.
With the recent opening of a new showroom in California, SED Pharma is now even more accessible to clients in the American market, enhancing its ability to serve a global clientele with top-tier equipment. This expansion, coupled with their commitment to worldwide shipping, underscores SED Pharma’s dedication to providing quality freeze-drying solutions globally.
